2025 Nobel Prize in Physiology or Medicine Announced: Immune System Breakthroughs Set to Broaden Prospects for Cell Therapy
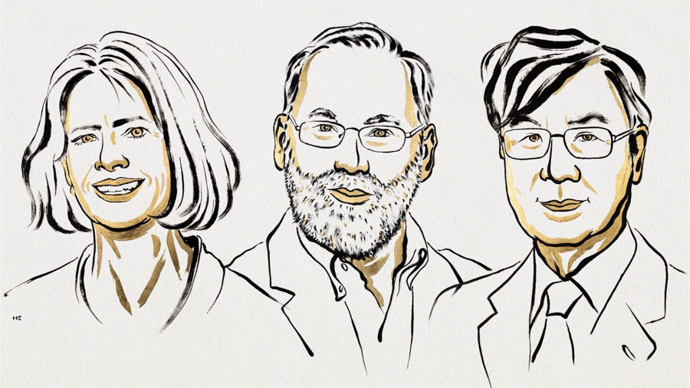
[Stockholm/Beijing, October 6, 2025] The Nobel Assembly at Karolinska Institutet announced that the 2025 Nobel Prize in Physiology or Medicine is awarded to Mary E. Brunkow (Institute for Systems Biology, Seattle), Fred Ramsdell (Sonoma Biotherapeutics, San Francisco), and Shimon Sakaguchi (Osaka University) for foundational contributions to peripheral immune tolerance and regulatory T cells (Tregs). “Their discoveries are decisive for understanding how the immune system maintains homeostasis and why not everyone develops severe autoimmune disease,” said Olle Kämpe, chair of the Nobel Committee.
Scientific milestones and mechanistic insights
In 1995, Shimon Sakaguchi systematically defined CD4⁺CD25⁺ regulatory T cells and demonstrated their capacity to suppress autoimmunity and maintain immune tolerance, opening the modern field of peripheral tolerance. In 2001, Mary E. Brunkow and Fred Ramsdell identified FOXP3 as the mutant gene underlying the lethal autoimmune phenotype of “scurfy” mice and linked human FOXP3 mutations to immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. In 2003, Sakaguchi and colleagues established FOXP3 as the master regulator of Treg development and function, consolidating the core framework of contemporary Treg biology. Collectively, these advances addressed a central question in immunology—how self versus non-self is enforced—and expanded the paradigm from thymic central tolerance to peripheral immune tolerance across cell subsets, transcriptional programs, and microenvironmental contexts.

Clinical and industry impact
Treg biology has advanced from basic science to translational strategies spanning autoimmune disease, organ transplantation, and cancer immunology. In oncology, reducing Treg-mediated suppression in the tumor microenvironment can enhance antitumor immunity; in autoimmunity and transplantation, ex vivo expansion or engineering of Tregs is being explored to restore or induce tolerance. Adoptive T-cell therapies such as CAR T-cell therapy are established in hematologic malignancies, while parallel efforts to develop Treg-based cell medicines and gene-regulation approaches continue to progress, with the number of related clinical studies steadily increasing.
Laureates and prize information
Mary E. Brunkow is a senior program manager at the Institute for Systems Biology (Seattle); Fred Ramsdell serves as a scientific advisor at Sonoma Biotherapeutics (San Francisco); and Shimon Sakaguchi is a chaired professor at Osaka University’s Immunology Frontier Research Center (IFReC). The laureates will share 11 million Swedish kronor, with the award ceremony scheduled for December 10 in Stockholm.

Significance and outlook
This year’s prize validates the peripheral tolerance–Treg–FOXP3 axis from cellular populations to molecular control, providing actionable levers for precision immune modulation. As robust methods for Treg expansion, purification, and engineering mature—and as the FOXP3 regulatory network is resolved in greater detail—these insights are expected to yield more predictable and reproducible clinical benefits in refractory autoimmune diseases, durable graft tolerance, and combination cancer immunotherapy.
